Estimating optimal vaccination coverage for goat plague eradication

To support the global initiative on eradicating peste des petits ruminants (PPR), also known as goat plague, veterinary expert from City University of Hong Kong (CityU) worked as part of an international research team to develop a dynamic model for estimating the optimal vaccination coverage required for eradicating this disease. They have found that rather than aiming for uniform vaccination coverage across the whole population in a particular country or region, the most effective way to eradicate the disease should involve targeting specific sub-populations acting as viral reservoirs.
PPR is an infectious disease of sheep and goats caused by a morbillivirus. It is highly transmissible among small ruminants, with morbidity and mortality risk up to 100% in populations without immunity. The disease is endemic across Africa, Middle East and large regions of Asia. There were outbreaks in Bangladesh, India and China in recent years. Luckily, the PPR virus does not infect humans. But it’s devastating impact on small ruminants is one of the main constraints to small ruminant production and welfare, and therefore a threat to food security and livelihoods of the poorest communities, for which sheep and goats are often an essential asset.
The World Organization for Animal Health (OIE) and the Food and Agriculture Organization of the United Nations (FAO) launched an initiative to eradicate PPR by 2030. The global strategy relies heavily on mass vaccination campaigns for small ruminant populations, based on the availability of an efficacious vaccine producing life-long immunity against all PPR virus serotypes after one administration.
However, the strategy recommending the vaccination of almost all small ruminants above 3 months of age is costly and difficult to implement in the field for a number of reasons, including the vaccine’s thermolability which needs to be kept under 8 degree Celsius, the logistical challenges of implementing large-scale vaccination campaigns, mobility of some small ruminant populations within and between countries, and the lack of precise census data and national animal identification systems. Therefore, it is essential to assess the spatial and temporal variation in PPRV transmission potential, so that sub-populations acting as a viral reservoir within geographical areas holding small ruminant populations can be targeted, and the minimal fraction of animals that needs to be vaccinated can be estimated.
Hence Dr Guillaume Fournié, Senior Research Fellow from the Royal Veterinary College (RVC) in London, has led an international research team with members including Professor Dirk U. Pfeiffer, Chair Professor of One Health, and Associate Dean (Research) at the College of Veterinary Medicine and Life Sciences, CityU, to develop a dynamic model.
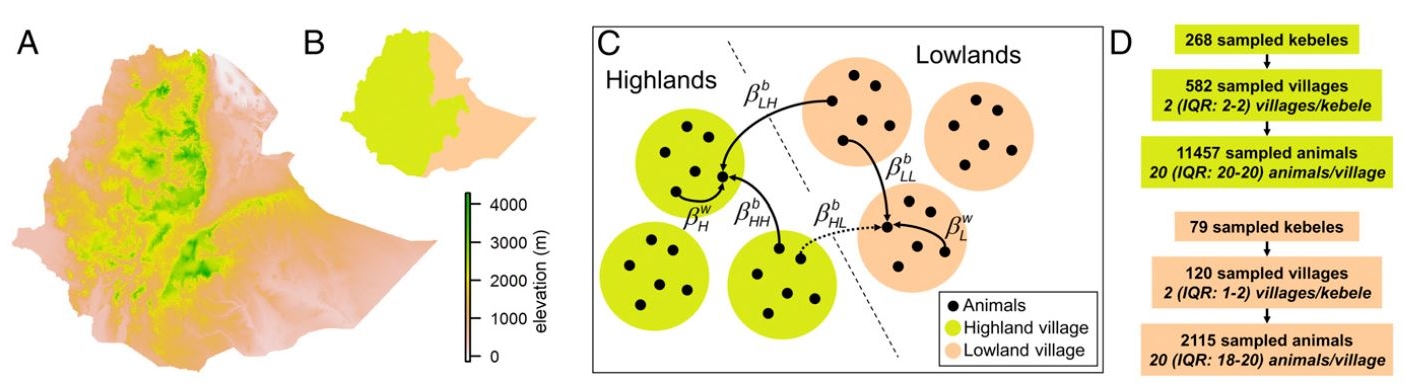
The researchers used Ethiopia as the case, which is one of the PPRV-endemic countries and has the world’s seventh largest small ruminant population. By using the results from a nationwide serological survey in Ethiopia to estimate the parameters of the dynamic model simulating viral spread, the team estimated that if the proportion of immune small ruminants is kept permanently above 37% in at least 71% of pastoral village populations, viral spread could be prevented in a particular geographical area in Ethiopia. And the results suggest that pastoral production systems keeping small ruminants which are common in many developing countries, in particular in Africa, should be targeted by repeated vaccination campaigns to reduce viral spillover to small ruminant populations kept in other production systems in Ethiopia and neighbouring countries.
The findings were published in the latest issue of academic journal Proceedings of the National Academy of Sciences (PNAS), under the title of “Transmission and elimination of peste des petits ruminants in Ethiopia: Insights from a dynamic model”.
“Although PPR is not a zoonotic disease, eradicating PPR is also of global ‘One Health’ relevance since it would contribute to improving food security in many countries, in particular within communities that rely on sheep and goats as one of the most important sources of meat or household income,” said Professor Pfeiffer.
“PPRV is only transmitted among a small number of ruminant species and the good vaccine is already available, so there is real potential to eradicate this disease globally. But we need to tailor our vaccination strategy to the epidemiological characteristics of this infectious disease in order to make this ambitious goal achievable. This will then mean that we will not have to aim for an unrealistically high vaccination coverage across all small ruminant populations, and instead focus our effort on targeting those parts of populations which are at highest-risk of transmission. That’s the key insight from this research,” he explained.
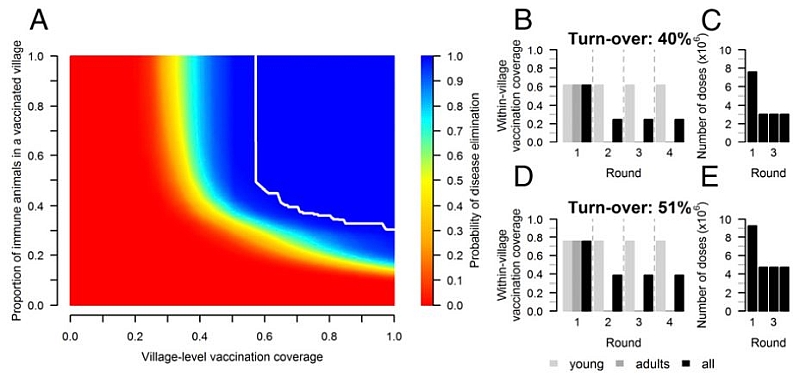
He added that although the data they used was from a nationwide serological survey in 1999, the conclusions will still apply in Ethiopia and the generic principles to other countries, and potentially even to other infectious diseases. Prof Pfeiffer also emphasized that as with any epidemiological model, the translation of the conclusions into policy should be performed cautiously, taking into account many other factors, including local knowledge. And for the model conclusions to inform national and regional components of the global strategy to eradicate PPR, it is important for the countries and global communities to have the necessary serological data from the field and to understand the local production system characteristics in order to define a targeted vaccination campaigns effectively.
The research is led by Dr Guillaume Fournié from the Royal Veterinary College, University of London. Other co-authors include Dr Agnès Waret-Szkuta from Université de Toulouse, Dr Anton Camacho from the London School of Hygiene and Tropical Medicine and Epicentre, the research arm of medical humanitarian organisation Médecins Sans Frontières, Dr Laike M. Yigezu from National Veterinary Institute in Ethiopia, and Dr Francois Roger from the French Agricultural Research Centre for International Development (CIRAD).