News Menu
Highly efficient novel electrocatalysts developed by CityU researchers to tackle energy crisis

Clean hydrogen energy is a good alternative to fossil fuels and is critical for achieving carbon neutrality. Researchers around the world are looking for ways to enhance the efficiency and lower the cost of hydrogen production, particularly by improving the catalysts involved. Recently, a research team from City University of Hong Kong (CityU) developed a new, ultra-stable hydrogen evolution reaction (HER) electrocatalyst, which is based on two-dimensional mineral gel nanosheets and does not contain any precious metals. The catalyst can be produced in large scale and can help achieve a lower hydrogen price in the future.
Electrochemical hydrogen evolution reaction (HER) is a widely used hydrogen-generation method. But commercial HER electrocatalysts are made from precious metals, which are expensive. On the other hand, single-atom catalysts have promising potential in catalytic HER applications because of their high activity, maximised atomic efficiency, and minimised catalyst usage. But the conventional fabrication process of single-atom catalysts is complicated. It generally involves introducing the targeted single-atom metal to the substrate precursor followed by thermal treatment, usually higher than 700 ℃, which requires a lot of energy and time.
In this regard, a research team co-led by CityU materials scientists have developed an innovative, cost-effective and energy-efficient way to produce a highly efficient HER single-atom electrocatalyst that uses precious-metal-free mineral hydrogel nanosheets as a precursor.
“Compared with other common single-atom substrate precursors, such as porous frameworks and carbon, we found that mineral hydrogels have great advantages for mass production of electrocatalysts due to the easy availability of the raw materials, simple and environmental-friendly synthetic procedure, and mild reaction conditions,” said Professor Lu Jian, Chair Professor in the Department of Mechanical Engineering (MNE) and the Department of Materials Science and Engineering (MSE) at CityU, who led the research.

? Lyu, F. et al (source: https://www.nature.com/articles/s41467-022-33725-8/)
Their electrocatalyst precursor is prepared using a simple method. First, solutions of polyoxometalate acid (PMo) and ferric ions (Fe3+) are mixed at room temperature, resulting in novel two-dimensional iron–phosphomolybdic-acid nanosheets. After excess water is removed by centrifugation, the nanosheets become mineral hydrogel free of any organic molecules. The process is much more convenient and economical than the previously reported processes that typically require high temperature and pressure, and longer time for the self-assembly of single-atom substrate precursors.
After a further phosphating treatment (at 500 ℃) of this mineral gel precursor, a single-iron-atom dispersed heterogeneous nanosheet catalyst (“Fe/SAs@Mo-based-HNSs”) is formed, avoiding the time-consuming fabrication process of loading single atoms on the substrate.
The experiments found that the new catalyst exhibits excellent electrocatalytic activity and long-term durability in the HER, manifesting an overpotential of only 38.5 mV at 10 mA cm?2, and ultra stability without performance deterioration over 600 hours at a current density up to 200 mA cm?2.
“This is one of the best performances achieved by non-noble-metal HER electrocatalysts,” said Professor Lu. “The unique idea of using mineral gel to synthesize monatomic dispersed heterogeneous catalysts provides an important theoretical basis and direction for the next step of scalable production of cheap and efficient catalysts, which can help contribute to lowering the cost of hydrogen production in the long run.”
Their findings were published in the scientific journal Nature Communications under the title “Two-dimensional mineral hydrogel-derived single atoms-anchored heterostructures for ultrastable hydrogen evolution”.
The first author of the paper is Dr Lyu Fucong from CityU. The corresponding authors are Professor Lu, Dr Li Yangyang, Associate Professor in MSE, and Dr Sun Ligang, Assistant Professor in the School of Science at the Harbin Institute of Technology.

? Lyu, F. et al (source: https://www.nature.com/articles/s41467-022-33725-8/)
The research was supported by the Shenzhen-Hong Kong Science and Technology Innovation Cooperation Zone Shenzhen Park Project, the National Key R&D Program of China, the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Science, Technology and Innovation Commission of Shenzhen Municipality, and the Hong Kong Innovation and Technology Commission via the Hong Kong Branch of National Precious Metals Material Engineering Research Center at CityU.
To tackle the high cost problem of commercial platinum-based electrocatalysts, the team led by Professor Lu made another breakthrough recently. They have provided a solution through the rational nanostructured alloy design to develop a low-cost, high-performance electrocatalyst.

? Liu, S. et al (source: https://www.science.org/doi/10.1126/sciadv.add6421)
Professor Lu’s team has been carrying in-depth research on alloy nanostructures which have both crystalline and amorphous phases simultaneously. They found out that the local chemical inhomogeneity, short-range order and severe lattice distortion in the nanocrystalline phase are desirable for application in catalysis, while the amorphous phase can offer abundant active sites with lower energy barrier for hydrogen evolution reaction. Therefore, they devoted their research efforts to designing and constructing dual-phased alloys to be excellent electrocatalysts for hydrogen production.

They proposed a new alloy and nanostructure design strategy which is based on thermodynamics. First, they predicted the composition range of the “crystal-amorphous” dual phase formation according to the amorphous forming ability (GFA). Then, using the facile method of magnetron co-sputtering, they successfully prepared the aluminium-based alloy catalyst with a “crystalline-amorphous” dual-phased nanostructure.
Thanks to this nanostructure, the new catalyst showed better electrocatalytic performance in alkaline solution than the commercial platinum-based electrocatalyst, with the overpotential of only 28.8 mV at 10 mA cm-2.
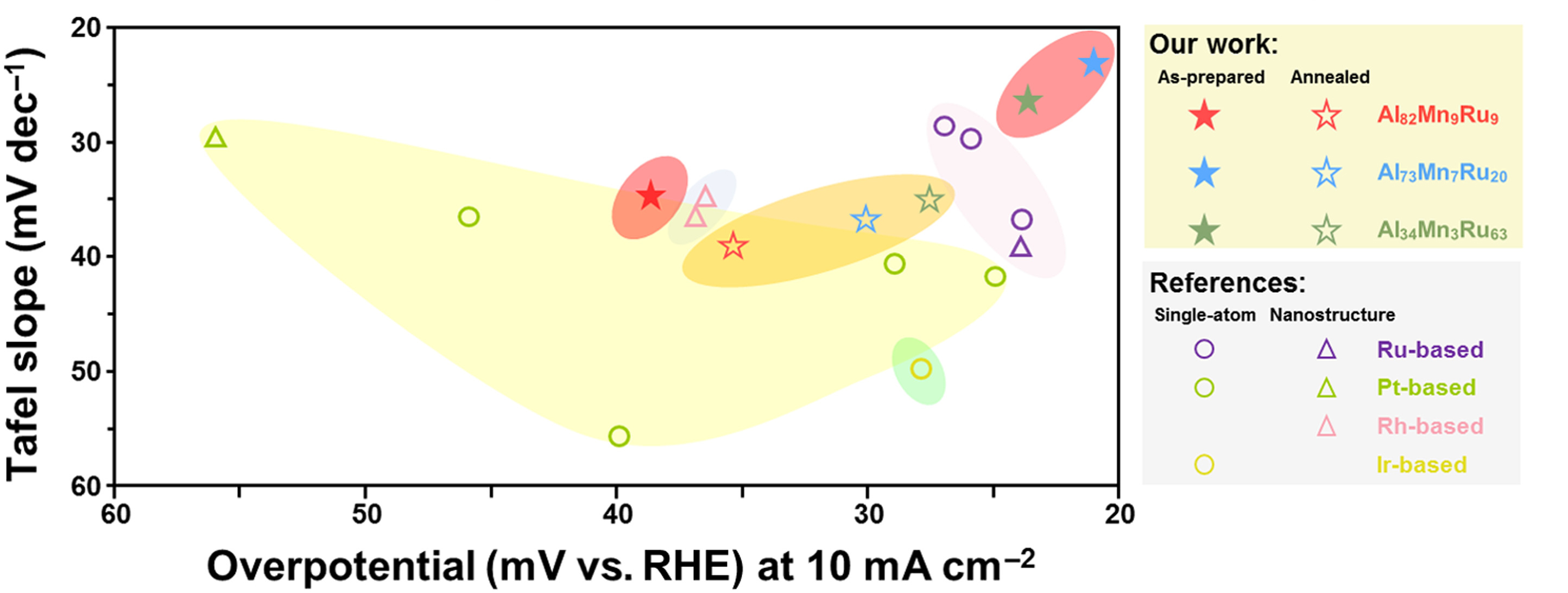
? Liu, S. et al (source: https://www.science.org/doi/10.1126/sciadv.add6421)
“In this novel aluminium-based alloy catalyst, we use ruthenium, which is cheaper than platinum, as the noble metal component. So it can be less costly than the commercial platinum-based electrocatalysts,” said Professor Lu. “And apart from hydrogen evolution, the nano-dual-phase electrocatalysis mechanism can be applied to other catalytic systems. The ‘crystal-glass’ nanostructure design offers a new approach to develop next-generation catalysts.”

The findings were published in Science Advances, under the title “A crystal glass–nanostructured Al-based electrocatalyst for hydrogen evolution reaction”. Dr Liu Sida, former postdoctoral fellow (currently a Professor at Shandong University), and Mr Li Hongkun, MSE PhD student, are the co-first authors. The corresponding authors are Professor Lu and Dr Li from CityU, and Professor Wu Ge from Xi'an Jiaotong University. Other researchers from CityU include Dr Zhou Binbin, a former postdoc at MNE (currently Research Associate Professor of Shenzhen National Institute of Advanced Electronic Materials Innovation), Mr Zhong Jing and Miss Li Lanxi, both are MSE PhD students, and Mr Yan Yang, MNE PhD student.